Infrared thermography
What it is ...
 Infrared Thermography is the technique that uses an infrared imaging and measurement camera to “see” and “measure” invisible infrared energy being emitted from an object. Thermal, or infrared, energy is energy that is not visible to the human eye because its wavelength is too long for the sensors in our eyes to detect. It is the part of the electromagnetic spectrum that we perceive as heat. Unlike visible light, in the infrared spectrum everything with a temperature above absolute zero emits infrared electromagnetic energy. Even cold objects such as ice cubes emit infrared radiation. The higher the temperature of the object, the greater the infrared radiation emitted. The infrared camera allows us to see what our eyes cannot!
Infrared Thermography is the technique that uses an infrared imaging and measurement camera to “see” and “measure” invisible infrared energy being emitted from an object. Thermal, or infrared, energy is energy that is not visible to the human eye because its wavelength is too long for the sensors in our eyes to detect. It is the part of the electromagnetic spectrum that we perceive as heat. Unlike visible light, in the infrared spectrum everything with a temperature above absolute zero emits infrared electromagnetic energy. Even cold objects such as ice cubes emit infrared radiation. The higher the temperature of the object, the greater the infrared radiation emitted. The infrared camera allows us to see what our eyes cannot!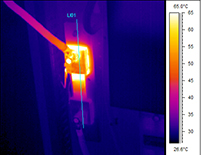 In the industrial/commercial environment, almost everything gets hotter or cooler before it fails, making infrared cameras extremely valuable diagnostic tools with many diverse applications. As industry strives to improve manufacturing efficiencies, manage energy, improve product quality and enhance worker safety, new applications for infrared cameras continually emerge. Likewise, buildings and structures have a myriad of components, all absorbing and emitting heat to the environment. Sometimes buildings “leak” heat – both inwards and outwards. To be able to “see” the difference in heat between the components can be invaluable so that interpretation of the thermograms may lead to conclusions regarding inappropriate heat loss/gain.
In the industrial/commercial environment, almost everything gets hotter or cooler before it fails, making infrared cameras extremely valuable diagnostic tools with many diverse applications. As industry strives to improve manufacturing efficiencies, manage energy, improve product quality and enhance worker safety, new applications for infrared cameras continually emerge. Likewise, buildings and structures have a myriad of components, all absorbing and emitting heat to the environment. Sometimes buildings “leak” heat – both inwards and outwards. To be able to “see” the difference in heat between the components can be invaluable so that interpretation of the thermograms may lead to conclusions regarding inappropriate heat loss/gain.How does the camera “see” heat?
All objects, cold or hot, radiate heat in the form of infrared energy. As an object increases in temperature, it radiates more energy and the wavelength gets shorter. Infrared radiation, visible light and ultraviolet light are all forms of energy in the electromagnetic spectrum. The only difference is their wavelength or frequency.

The human eye can only see a narrow range of wavelength in the electromagnetic spectrum. These wavelengths range in length from 0.4 to 0.7 microns (a micron is one millionth of a metre). Most of what the eye sees is reflections from objects that high energy from the sun or an incandescent light bulb is striking. If the temperature of an object gets hot enough, however, above 525°C the energy from that object will radiate energy in the visible spectrum and we will see it. This is when we see an object like the burner on an electric stove “glowing” red. In fact any time an object will emit or reflect energy in the same frequency of our eyes we will see it. Mostly, however we see reflections.
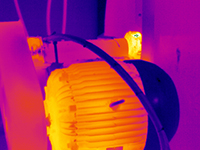 The infrared camera can detect infrared energy well before we can see it with our eyes. Most cameras can image temperatures from -20 to 500°C and can be extended down to -40°C and up to 2000°C. The camera converts this invisible infrared energy into a two-dimensional visual image and displays this on a standard TV monitor. Most industrial cameras can also make temperature measurements, with accuracies to around ±2% at 30°C. The thermal information is stored on to a disc and is later downloaded into a computer to create a report.
The infrared camera can detect infrared energy well before we can see it with our eyes. Most cameras can image temperatures from -20 to 500°C and can be extended down to -40°C and up to 2000°C. The camera converts this invisible infrared energy into a two-dimensional visual image and displays this on a standard TV monitor. Most industrial cameras can also make temperature measurements, with accuracies to around ±2% at 30°C. The thermal information is stored on to a disc and is later downloaded into a computer to create a report.Taking thermal images and gathering thermal information is quite easy these days, just push the auto button and there is an image! This is simple on the surface, but it is not as easy as it sounds. The real work — and value — is what the thermographer understands about the object of interest, how it operates, the heat transfer within and to the surface of the object and how to adjust the camera to enhance the thermal details necessary to evaluate the image once it is stored and downloaded on to the computer. Then it is usually necessary to prepare a report that is accurate, clearly presented and easy to read by the maintenance personnel, who generally do not know anything about infrared thermography. As in any method of non-destructive testing, the interpretation of the information gathered takes both education and experience.
A good thermographer must thoroughly understand all the variables.